Research
Graduate Research
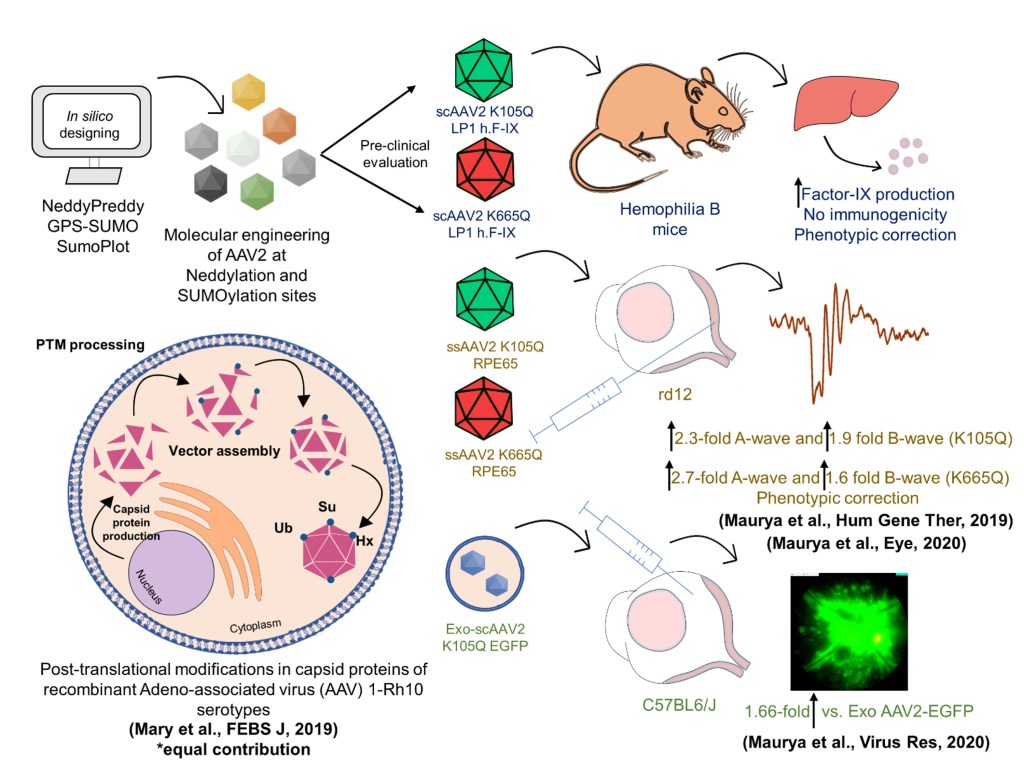
During my Ph.D. at the Indian Institute of Technology (IIT) Kanpur, my research focused on developing innovative gene therapy vectors to treat hemophilia B, a bleeding disorder, and Leber congenital amaurosis (LCA2), a retinal degenerative disease. Gene therapy relies on adeno-associated virus (AAV) vectors to deliver therapeutic genes to target cells, but their efficiency is often hindered by modifications inside the cell that degrade the viral particles. To address this, I identified and modified specific sites on the AAV capsid proteins, enhancing their ability to deliver genes effectively. These engineered vectors successfully corrected disease phenotype in mouse models, restoring vision in LCA2 and improving clotting function in hemophilia B. In a related study, I worked on mapping the cellular modifications affecting AAV vectors using advanced LC-MS/MS proteomics tools, identifying key sites across multiple AAV serotypes. Additionally, I developed a novel approach to package AAV vectors within exosomes, naturally occurring cell-derived particles, to reduce immune responses and improve the safety of gene therapy. Beyond retinal diseases, I collaborated on a project using exosomes to deliver “suicide genes” for cancer therapy, which significantly reduced tumor size in a mouse model of liver cancer. These efforts led to three Indian and two U.S. patents, several of which are now licensed to industry collaborations and being developed as India’s first indigenous gene therapy platform.
Postdoctoral Research
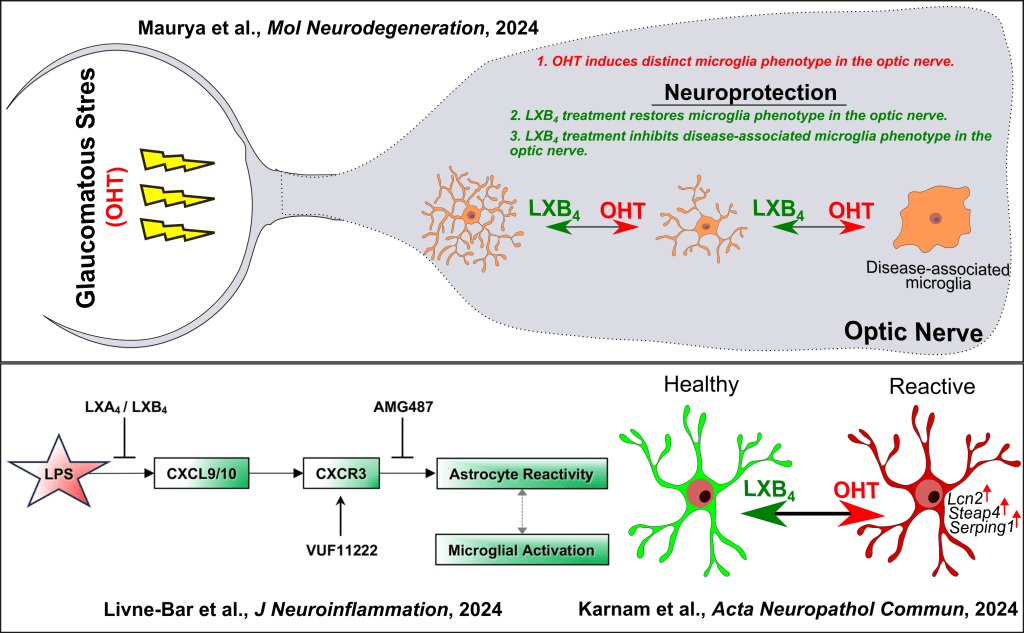
As a postdoctoral fellow in the Gronert Lab at the University of California, Berkeley, my research focuses on studying the neuroprotective role of Lipoxin B4 (LXB4), a lipid molecule identified by the lab that helps protect retinal ganglion cells (RGCs) in the retina from glaucomatous stress. My goal was to understand how LXB4 works and which cells it targets. Through single-cell transcriptomics and morphOMICs, I discovered that microglia, immune cells in the retina and optic nerve, are key targets of LXB4. Microglia play a crucial role in maintaining healthy microenvironment but can become inflamed during glaucomatous stress, contributing to neurodegeneration. Next, I further found that microglia in the optic nerve respond differently to glaucomatous stress than those in the retina. Specifically, glaucomatous stress induces a unique disease-associated microglia in the optic nerve, marked by a protein called CD74, which was not found in the retina. Treatment with LXB4 shifted these inflamed microglia back to a healthy state, revealing a novel mechanism by which LXB4 protects the optic nerve. This work highlights the importance of targeting microglia to develop treatments for glaucoma and other neurodegenerative diseases. In collaboration, we showed that lipoxins inhibit glial cell activation via the CXCR3 signaling pathway in an acute retinal neuroinflammation model induced by bacterial lipopolysaccharide. Additionally, we established the presence and relevance of the lipoxin pathway in the mouse retina, macaque optic nerves, and human astrocytes using LC-MS/MS lipidomics approach, highlighting its dysregulation in human astrocytes during glaucomatous stress.